Abstract
Background:
Chimeric antigen receptor T-cell (CAR-T) therapies directed against B-cell receptor maturation antigen (BCMA) have elicited impressive responses in patients with relapsed/refractory multiple myeloma (RRMM), leading to the FDA approval of two BCMA-targeted CAR-T therapies. Despite high response rates, the majority of patients treated with BCMA CAR-T relapse and the efficacy of current salvage therapies after relapse is limited, highlighting the need for development of new treatment strategies.
There has been increasing evidence that the immunosuppressive effects of the tumor microenvironment may play an important role in resistance to CAR-T therapy. Programmed death-1 (PD-1) is one of the co-inhibitory receptors that is upregulated following antigen exposure and is associated with T-cell exhaustion. Previous studies have demonstrated increased levels of PD-L1 on multiple myeloma cells along with increased PD-1 expression in T-cells in patients with treatment-resistant disease.1-2 In pre-clinical studies, antibody therapy targeting the PD-1/PD-L1 axis has been able to abrogate the immunosuppressive effects of the tumor microenvironment, increasing levels of tumor-specific CD8+ T-cells and inhibiting tumor growth.2-3 Therefore, one hypothesis is that PD-1 blockage may restore or augment the potency of tumor-specific exhausted CAR-Ts. This proof-of-concept was recently demonstrated by Chong et al. in a study of pembrolizumab, an anti-PD1 antibody, in the treatment of patients with B-cell lymphomas relapsing or refractory to prior anti-CD19 CAR-T therapy; 4 of 12 patients treated on trial re-expanded CAR-Ts measured by mass cytometry by time of flight and this translated to a clinical overall response rate (ORR) of 25%.4
In our study, we aim to investigate the safety and efficacy of pembrolizumab in RRMM patients who have relapsed or developed refractory disease after anti-BCMA CAR-T therapies.
Study Design and Methods:
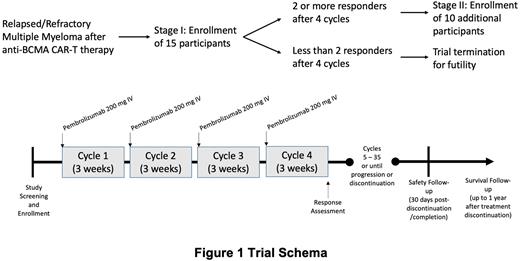
This is a Phase II, single-center study of pembrolizumab for the treatment of RRMM after anti-BCMA CAR-T therapy. The study population will include adults ≥ 18 years or older who develop progressive disease after prior anti-BCMA CAR-T therapy and have not started another systemic anti-cancer therapy following progression. Time from date of anti-BCMA CAR-T infusion to date of first administration of pembrolizumab must be ≥ 90 days. Participants must have previously received or are intolerant to a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody.
Eligible patients will receive pembrolizumab 200 mg intravenously, every 3 weeks for up to 2 years (35 cycles) or until disease progression/dose-limiting toxicities/discontinuation. The study utilizes a Simon's two-stage design with 15 participants enrolling in stage I and 10 additional participants enrolling in stage II assuming that at least 2 or more participants achieve a treatment response after 4 cycles of therapy during stage I of the study. The primary endpoint will be ORR (partial response or better by IMWG criteria) after 4 cycles of therapy. The Simon's minimax two-stage design will be used to test the null hypothesis that the true ORR is 10% against a one-sided alternative that the true ORR is higher than 30%. The two-stage minimax design will have an 80% power to reject the null hypothesis that ORR is 10% at one-sided Type I error rate of 5%, assuming that the ORR is 30%.
Secondary analysis endpoints include toxicity, depth of response, overall survival, progression free survival, and time-to-next treatment. A Bayesian toxicity monitoring design will be incorporated for ongoing safety analysis to pause enrollment if at any time the rate of grade 3 or higher non-hematologic adverse events has a high posterior probability exceeding 30%. Toxicities of interest include cytokine release syndrome and immune-effector cell-associated neurotoxicity. Exploratory analysis includes assessing the immune profile after pembrolizumab treatment, including changes in absolute lymphocyte count and lymphocyte subsets by flow-cytometry.
This ongoing study (NCT05191472) is currently open and enrolling at University of California, San Francisco.
References:
1. Paiva B et al. Leukemia. 2015; 29(10):2110-3.
2. Hallett W et al. Biol Blood Marrow Transplant. 2011;17(8):1133-45.
3. Jing W et al. J Immunother Cancer. 2015;3(1):2.
4. Chong E et al. Blood. 2022; 139(7): 1026 - 1038.
Disclosures
Chung:Sanofi: Honoraria; Caelum: Research Funding; Merck: Research Funding; AbbVie: Research Funding; BMS/Celgene: Research Funding; Cellectis: Research Funding; Janssen: Research Funding. Martin:Legend Biotech: Consultancy; GlaxoSmithKline and Legend Biotech: Consultancy; Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding. Wolf:Adaptive Biotechnologies: Consultancy. Wong:Patient Discovery: Research Funding; Dren Bioscience: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees; Caelum: Research Funding; Bristol Myers Squibb: Research Funding; Fortis: Research Funding; Genentech: Research Funding; Janssen: Research Funding; GSK: Research Funding; Catalent Biologics: Consultancy. Shah:Kite: Consultancy; Poseida: Research Funding; Nektar: Research Funding; BMS/Celgene: Consultancy, Research Funding; Precision Biosciences: Research Funding; CSL Behring: Consultancy; Allogene: Consultancy; Sutro Biopharma: Research Funding; Karyopharm: Consultancy; Sanofi: Consultancy; Janssen: Research Funding; TeneoBio: Research Funding; AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Bluebird Bio: Research Funding; Indapta Therapeutics: Consultancy; Amgen: Consultancy; CareDx: Consultancy; GSK: Consultancy; Oncopeptides: Consultancy.
OffLabel Disclosure:
The use of pembrolizumab for treatment of multiple myeloma after CAR-T relapse.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal